.
The Hydrocarbons:
A Brief Breeze Through
Hydrocarbons are always present in the daily lives of the average working class, for one reason; transportation. You see, hydrocarbons are present in many things but most importantly it is included in fuels, and what do fuels do? they give power to a vehicle, fuels give vehicles the power to transport a person or a group of people from one place, to the next and so much more! So what's in these fuels? Let's find out.
Origin
Sourced from crude oil and raw natural gas. Aromatic Hydrocarbons are present in the nucleic acids of the human body such as the DNA and amino acids. Aliphatic hydrocarbons are composed of catenated carbon chain.
Classifications
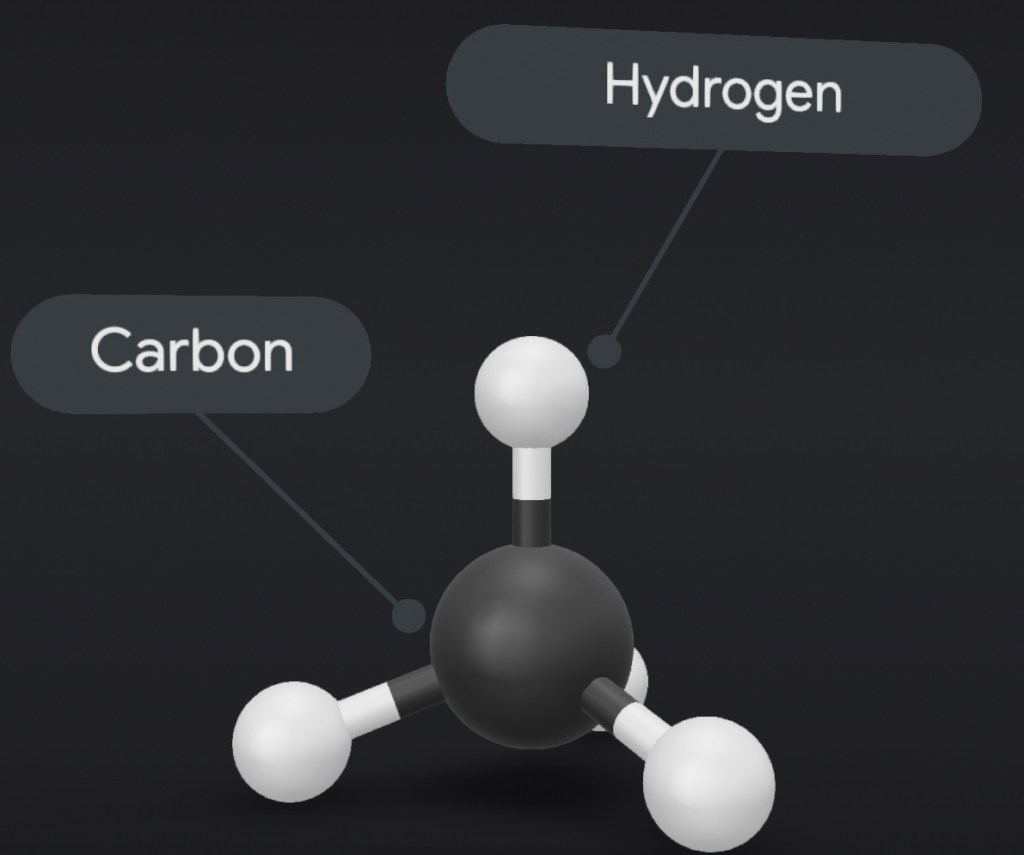
Hydrocarbons are made of two atomic elements, carbon and hydrogen, therefore they are classified as organic compounds.
Types
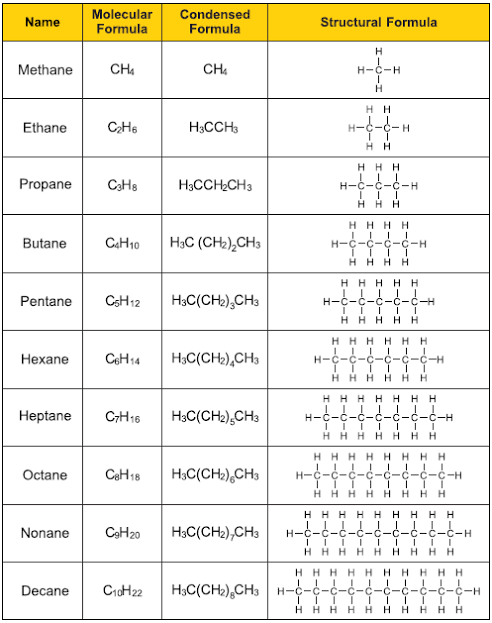
There are two types of hydrocarbons: aliphatic and aromatic. The three types of aliphatic hydrocarbons are alkanes, alkenes, and alkynes. Aromatic hydrocarbons include benzene.
Gallery

Structure
Alkanes
In organic chemistry, an alkane, or paraffin is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds aresingle.[1] Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane (CH4), where n = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (C14H30).
In an alkane, each carbon atom is sp3-hybridized with 4 sigma bonds (either C–C or C–H), and each hydrogen atom is joined to one of the carbon atoms (in a C–H bond). The longest series of linked carbon atoms in a molecule is known as its carbon skeleton or carbon backbone. The number of carbon atoms may be considered as the size of the alkane.
Benefits
Alkanes are important raw materials of the chemical industry and the principal constituent of gasoline and lubricating oils. Natural gas mainly contains methane and ethane and is used for heating and cooking purposes and for power utilities (gas turbines). For transportation purposes, natural gas may be liquefied by applying pressure and cooling it (LNG = liqid natural gas). The Sultanate of Oman, for example, exports most of its natural gas as LNG – see the LNG plant at Qalhat which has been designed to liquefy 6.6 million tons natural gas per year. Crude oil is separated into its components by fractional distillation at oil refineries. The different “fractions” of crude oil have different boiling points and consist mostly of alkanes of similar chain lengths (the higher the boiling point the more carbon atoms the components of a particular fraction contain)
Risks
Swallowed, liquid alkanes do little harm while in the stomach. In the lungs, however, they cause “chemical” pneumonia by dissolving fatlike molecules from cell membranes in the tiny air sacs (alveoli). The lungs become unable to expel fluids, just as in pneumonia caused by bacteria or viruses. People who swallow gasoline or other liquid alkane mixtures should not be made to vomit, as this would increase the chance of getting alkanes into the lungs. (There is no home-treatment antidote for gasoline poisoning; call a poison control center.) When alkane fuels combust the following pollutant occur: Carbon dioxide • Carbon monoxide • Carbon • Sulfur dioxide • Oxides of nitrogen • Unburned hydrocarbons These pollutants are responsible for a number of environmental problems, including acid rain, global warming and smog.
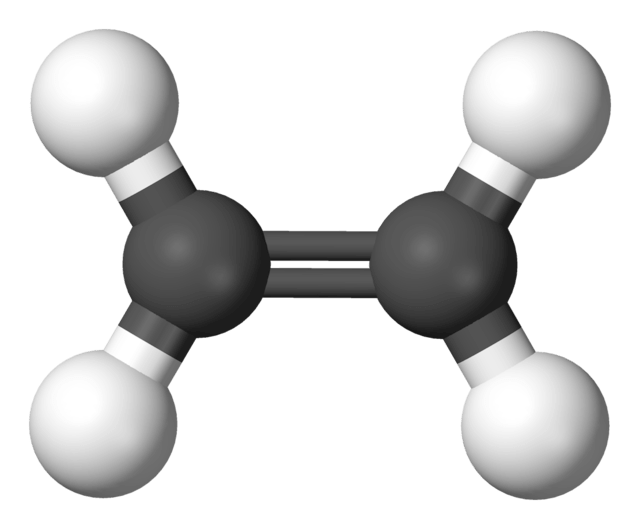
Alkenes
Definition: Alkenes or olefins (as used by the petroleum industry) is a family of Hydrocarbons consisting of:
• Ethene (C2H4)
• Propene (C3H6)
• Butene (C4H8)
• Pentene (C5H10)
• Hexene (C6H12)
• Heptene (C7H14)
• Octene (C8H16)
• Nonene (C9H18)
• Decene (C10H20)
• Undecene (C11H22)
with the general formula of (CnH2n). Alkene is more reactive than Alkanes due to the presence of at least one carbon-to-carbon double bond (C=C) this carbon-carbon double bond changes the physicals properties of alkenes. At room temperatue, alkenes exist in all three phases, solid, liquids, and gases. Alkenes contain less than the maximum possible number of hydrogen atoms per carbon atom, they are said to be unsaturated.
Benefits
They are used in daily life to make it more… efficient, They are important feedstock for series of industrial chemicals specially polymers. Alkenes are used in the synthesis of alcohols, lacquers, detergents, and fuels as starting materials. For the chemical industry, the most important alkenes are ethene, propene, and 1,3-butadiene. Alkenes are the raw materials for plastics such as polyethene, PVC, polypropylene, and polystyrene, among others.
Risks
Though there is no doubt that these Hydrocarbons helped us quite a lot, they also pose a risk to us, Alkene is quite flammable and a certain other type-2 alkene derivatives are highly toxic by-products of membrane lipid peroxidation associated with cellular oxidative stress.
Side Note: The terms alkenes and olefins often are used interchangeably; however, this is not quite accurate. According to IUPAC, alkenes include all aliphatic hydrochloride exhibiting one and only one double bond. Olefins encompass a larger set of compounds. As a matter of fact, olefins include all aliphatic (both acyclic and cyclic) hydrocarbons having one or more carbon-to-carbon double bonds: cycloalkenes, alkenes, and polyenes (compounds exhibiting more than one double bond). When an alkene has more than one double bond, the nomenclature changes to alkadiene, alkatriene, and so on.
Structure
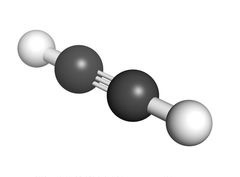
Alkyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
Benefits
Alkynes can be a useful functional group to synthesize due to some of their antibacterial, antiparasitic, and antifungal properties. One simple method for alkyne synthesis is by double elimination from a dihaloalkane.
Alkynes and compounds containing alkynes in their chemical structures are useful in various industries. For instance, in the fuel industry and plastics industry, alkynes like propyne and acetylene are used as starting materials in manufacturing plastic products.
It is also used for making polymers and it is a precursor. For instance, vinyl chloride is used as the starting material for PVC and chloroprene is used for synthetic rubber neoprene. Ethyne is used for preparing many organic solvents. Alkynes are commonly used to artificially ripe fruits.
Risks
With alkanes, alkenes and alkynes the primary hazard is flammability. The vapors of these compounds may be lighter or heavier than air among the gases and heavier than air with the liquids. Most flammable liquids have a specific gravity less than 1 and will float on water. Alkynes are relatively acidic which can create a powerful and dangerous explosion. It also tends to be more reactive than alkanes and alkenes.
Structure
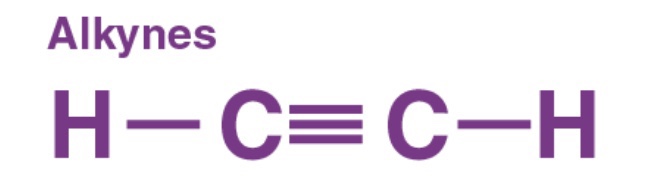
Questions
1. What kind of reactions do alkanes undergo?
The two most important reactions of alkanes are combustion and halogenation. Combustion: Alkanes undergo a combustion reaction to give carbon dioxide and water. Halogenation: Alkanes undergo halogenation reaction to give haloalkane.
2. What is an example each of combustion reaction and halogenation reaction of alkanes?
Combustion reaction: Methane undergoes combustion reaction to give carbon dioxide and water. CH4 + 2O2 → CO2 + 2H2O
3. What are the characteristics of alkenes?
Some of alkenes and alkanes’ physical properties are similar: they are colourless, nonpolar, and combustible. Alkenes are lighter than water. Alkenes are insoluble in water and soluble in organic solvents such as benzene. The boiling points of alkenes show a gradual increase with an increase in the molecular mass or chain length, this indicates that the intermolecular attractions become stronger with the increase in the size of the molecule.
4. Can alkenes be used as fuel?
Unlike alkanes, alkenes burn readily to give carbon dioxide and water if, for example, the combustion is complete. Despite two reasons, however, they are NOT used as fuels. To make plastics, anti-freeze and numerous other useful compounds, they are far too valuable for use.
5. What kind of reactions do alkynes undergo?
Alkynes undergo addition reactions due to the presence of loosely held pi-electrons. Due to the presence of a triple bond in alkynes, halogens, water etc. can be added to them by the process of the addition reaction. Alkynes and halogens undergo addition reaction to form halogenated alkenes which further react with halogens to give halogen substituted alkanes. The reddish orange coloured solution of bromine and carbon tetrachloride gets decolorized as a result of the addition reaction. This is used as a test for unsaturation.
6. Give an example each of combustion reaction and halogenation reaction of alkynes.
In the alkyne there are one sigma bond and two pi bonds between two carbon atoms. These pi bonds are electron rich species; they form bonds with electrophile to form electrophilic addition reactions. Example: halogenation, hydration reaction.
What Do You Think?
Feel free to ask us whatever you want in; https://hydroc4rbon.talkyard.net/-12/ask-whatever
